Introducing BSI’s mAb panel for complement therapy development.
At BSI we have created a panel of over 150 epitope-specific mAbs against complement proteins using our protein epitome profiling technology platform. Many of them have the potential to be used in therapy development.
Characterization of our mAb panel
The components of our QuantiplasmaTM mAb library were characterized in collaboration with the research group of Prof. Mihaly Jozsi, MTA-ELTE Complement Research Group, Department of Immunology, Eötvös Loránd University, Budapest, Hungary.
Approach
- Pull down followed by deep MS analysis
- Western blotting
- Cognate proteins tested in recombinant or purified form
- Mimotope peptides (from phage display)
Over 150 epitope-specific mAbs to complement proteins
Our library contains 156 epitope-specific mAbs to complement proteins, of which 42 have shown inhibition in our in vitro assays. Many are potentially suitable for therapy development.
We target: C1q, C3, C4, C5, C6, C7, C8, C9, C4BP, Protein S, FH
Two examples: inhibition of alternate complement pathway-mediated hemolysis
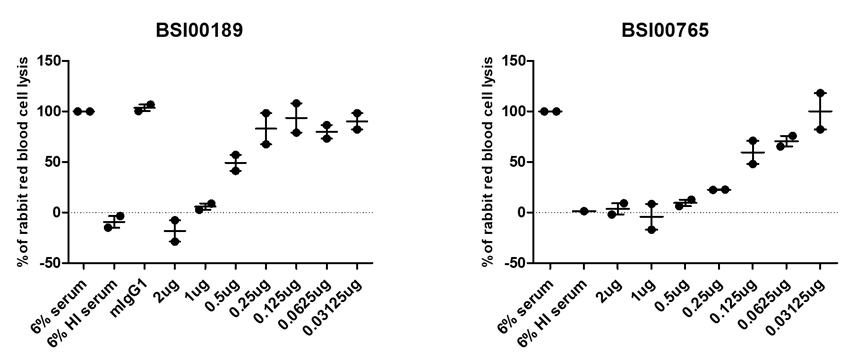
Next steps
- Extended in-vitro validation
- In-vivo validation
- Humanization, affinity maturation
- In-vivo & in-vitro validation of humanized mAbs
- Preclinical
- Clinical R&D
What we offer
1. Out-licensing
Individual mAbs or groups of mAbs for complement therapeutic development
2. Monoclonal antibody epitope panel development
Selected targets and groups of targets
3. Specialty complement-specific ELISA development
Unique features:
- More than 150 different mAbs available
- 42 mAbs showing an inhibitory effect
- Multiple mAbs for one complement protein due to epitope- level differentiation
- Native proteins used for mAb development (no recombinant proteins or peptides)

